Navigate: E-ssentials is a collection of e-learning modules covering a variety of topics presented in other Navigate offerings. While they are very useful resources and provide an overview of variety of research administration topics, they are not meant as a replacement for attending the instructor-led sessions. A large portion of those courses involve discussion-based, experiential learning activities that are not included in the e-learning courses.
View instructor-led course descriptions.
View Navigate: E-ssentials e-learning course descriptions.
View Lunch & Learn descriptions.
Please contact us at navigate-research@umich.edu. Much of what we offer is driven by feedback and suggestions from the U-M research administration community. We are always looking for volunteers to serve in various capacities (trainers, content contributors, committee members, etc.).
We ask that you notify us as soon as possible if you are not able to attend a course in which you've been accepted. Participants are not charged until after the course begins. If you have to miss a day of class due to unforeseen circumstances, we can usually work with you to make up the session at a future offering.
While Navigate courses are offered throughout the year, most are usually held in the Fall and Spring. Instructor-led courses are usually offered two-three times per year.
All course offerings are announced via the RAP/RAPid newsletter.
Most instructor-led courses require multiple, full-day commitments on the part of the participant, in addition to time outside of class to complete associated homework assignments. We must ensure that your supervisor is aware of and agrees to your full participation in the course and the time requried to participate.
Most instructor-led courses are held at Wolverine Tower. Lunch & Learn sessions are often held at various locations around campus.
Course fees vary. Specific information can be found on the course description pages.
Navigate instructor-led courses include a lecture component, experiential activities, discussions, and Q&A. Navigate courses are delivered via Zoom meetings and participants are given access to an accompanying Canvas course to locate and download course materials, resources, and job aids. More information about each course can be found on the instructor-led courses page.
All Navigate courses are designed by a team of highly-experienced U-M research administrators and central office staff in collaboration with instructional designers from the Navigate team.
All instructor-led courses are taught by highly-experienced U-M research administrators who have volunteered to teach on topics with which they have extensive knowledge/experience.
When a new course offering is announced, there will be a link to the application form on the course page. The course application differs depending on the specific course. Make sure to sign up to receive the RAP/RAPid newsletters to receive course announcements.
All members of the U-M research administration community are eligible. Some instructor-led courses have additional technical or experiential requirements. View the Navigate instructor-led course descriptions for specific requirements.
The Navigate program provides training and resources including instructor-led courses, eLearning, webinars, mentoring, job aids, and other information for the U-M research administration community. The program is sponsored by the Offices of Research and Sponsored Projects (ORSP) and Finance - Sponsored Programs.
Please direct inquiries to UMOR.HR.Team@umich.edu.
Because, if they are paid with sponsored funds (codes 20000 and 25000), then they must abide by the terms of sponsor agreement. They also may report time differently, and not use the Time and Labor System.
Read the sponsor guidelines, the funding opportunity, and the provisions of the award. The Principal Investigator may need to contact sponsor which may have requirements to notify them or seek their approval for a change in plans related to a leave.
- Typically sponsors require notification. Seek their approval for reducing planned effort by >25% or for time away from the project for > 3 months for key personnel and possibly complete a Post-Award Change Request (PACR).
You must work with your principal investigator to take the necessary steps and work with the sponsor. Prior approval is needed. You may need to initiate a Post-Award Change Request form.
These details must be arranged with the principal investigator and the sponsor and may require prior approval, initiated through use the Post-Award Change Request form. Read the sponsor guidelines, the funding opportunity, and the provisions of the award.
Refer to Human Resources Website and SPG 201.30-6 for more information on these policies. In short, the new maternity leave benefit supports a standard 6-week recovery period (up to 240 hours with a full-time appointment). In the event of a cesarean section or medically complicated delivery, extended sick time or other time off may supplement maternity leave. In addition, there is a 6-week parental leave for bonding.
Please visit the HR website and SPG 201.30-6 for comprehensive information on these policies and related HR processes. For quick reference, see the Summary Guide of Time-off Options for New Parents.
These questions relate to maternity and parental leave and are handled by U-M Human Resources. They have policies in place. Please visit the HR website and SPG 201.30-6 for more information on these policies and related HR processes. Also see the Summary Guide of Time-off Options for New Parents.
Yes. The OVPR pool is intended to provide support for those on sponsored funds as well as all postdoctoral research fellows, funded through other means (e.g., faculty discretionary, departmental ). Follow the steps above, “For Those Who Need a Leave.”
Attachments --> AWD Docs
Manage Unit Documents --> Unit Docs
Activity History --> Activity Log
Once your study is registered and issued an NCT number, you need to add the NCT number to the study workspace in eResearch Regulatory Management. Anyone who has edit rights on the study in eResearch can add the NCT number (no IRB staff involvement is required). If your IRB Application is for a multi-site trial, refer to the eResearch instructions regarding your responsibility for registering the study at ClinicalTrials.gov.
To add the NCT number:
- Click the “Update NCT Number” on the left sidebar and enter the NCT number in the box provided.
- An 8-digit number is required – including 1 or more zeros at the beginning.
No, trial results are not required for "non-applicable" trials that are registered on ClinicalTrials.gov solely to comply with ICMJE policy.
It can take anywhere between 20-40 hours to submit results on ClinicalTrials.gov.
Failure to register/update a trial/study or providing incomplete, false or misleading registration information may result in:
- Monetary penalties
- Withholding of federal research funds
- Return of grant funds to the sponsor
- Refusal of consideration by ICJME member (or other) journals
You should plan for registration to take one to two hours. Also note, that the PRS review process can take a few days, so plan accourdingly to ensure the study is registered on the public facing site within the required time-frame.
The Responsible Party may submit a certification or extension request in ClinicalTrials.gov to request a delay for reporting results.
- Certification requests are allowable when the study is completed before a new drug or device is initially approved, licensed, or cleared by the FDA, or when the manufacturer/sponsor of the study is applying for approval/clearance of a new use of an already approved drug or device. An approved certification request will delay the deadline to report results for up to two years from the certification date or for up to 30 days after the FDA approves, licenses, or clears the drug or device, whichever occurs first. Only one certification delay is allowable per study.
- Extension requests are allowable for a yet-to-be-defined list of "good cause." The NIH reviews each request and grants reporting extensions.
The eResearch Proposal Management (eRPM) team, along with leaders and consultants around campus, helped enhance eRPM with new Award Management functionality in August of 2018.
To start an Award Change Request (ACR), use the Request Action/Modification activity in the eRPM Award record and upload a signed copy of the Post Award Change Request (PACR) form to request changes to the award. Subsequent changes in terms and conditions, additional funding, time extensions, and other modifications may require ORSP staff to complete a Modification (MOD) to the AWD record in eRPM. Completed ACRs and MODs can be reviewed and accessed through the Modifications tab in the AWD record.
Every award is different. You must read the Award, and any details in the Sponsor Guidelines to understand how and when you will receive the funds. You need a P/G number set up to start spending. After you’ve reviewed the award you can contact your assigned Finance - Sponsored Programs Customer Service Representative with questions.
Should the PI want to modify the budget distribution, the proposed changes may be made on a Budget Reallocation Form, which should then be routed through the normal approval process.
The AWD record in eRPM contains attachments and details including the terms and conditions, key personnel, and other items that the project team can review.
At the time of award activation, a system-generated email notification is sent to all investigators and administrative personnel listed on the award and identified email contacts for all departments participating in the award informing them that the AWD has been created in eRPM. That notice provides the AWD number and the Project/Grant (P/G) number for the project.
If you have already consulted the helpful NIH Public Access site, and are still unable to come into compliance, the U-M Library staff may be able to assist. Send an email request for assistance to: nihms-library-support@umich.edu.
Yes, it will show up as Hardship. The Project/Grant will not have an award ID until it is awarded.
Today, ORSP adds commitment lines in the PAF. In Award Management, ORSP will complete an award modification to add new funding information to an award. The new budget line information will appear on the $$$ tab in the Award workspace.
A link to M-Reports is available on the Main tab in Award Management. You can use it to locate more detailed information about the Project/Grant, including any subs under the parent.
PANs and PACs are available on legacy (pre-Award Management) PAFs under Manage Data > View All Award Notices on the PAF workspace.
No. PAF comments will not be migrated over to the Award.
Post-award hardship requests will be submitted through a new award change request type available both on the PAC-R form and the online Award Change Request.
When Award Management goes live in August, fully automated routing of unit requests will not be in place but is planned for a future system upgrade.
Until then, the PI/Project Team will have two options to request an award change:
- Complete the current PACR form, obtain ink signatures, and upload the form using the new Request Action/Modification activity available in the Award workspace
Or - Use the new online Request Action/Modification activity
- Fill out the online Request Action/Modification form, print the form, and obtain ink signatures
- Upload the signed form to your change request and click Submit
No. The PAF exists for historical purposes. Once a PAF is funded, it is for reference only.
All modifications are handled on the award. When a modification is made to the award, the PAF will not change. Links to modifications display on the Mod/ACR tab in the Award workspace.
All PAFs tied to a single award will show up on the Related Records tab. From this tab, you can link back to the PAF if needed.
eRPM will be unavailable during the weekend of the system upgrade and data conversion. Tentatively, the temporary system outage will run from 5:30 p.m. on Friday, August 17 through 6:00 a.m. on Monday, August 20.
eRPM system users (unit and central offices) will be notified in advance of the weekend for the upgrade once the dates are confirmed to allow for planning.
 University of Michigan Supports Adoption of Accelerated Clinical Trial Agreement (ACTA). For those industry partners that agree to use the agreement (and the sponsor must agree to use it), it will decrease contract negotiation time. The other steps, such as budget negotiation, proposal approval form (PAF) processing, and IRB review and approval are still required. And U-M is committed to exploring process improvements and working toward the goal of reducing the total time to opening a clinical trial in all of these areas.
University of Michigan Supports Adoption of Accelerated Clinical Trial Agreement (ACTA). For those industry partners that agree to use the agreement (and the sponsor must agree to use it), it will decrease contract negotiation time. The other steps, such as budget negotiation, proposal approval form (PAF) processing, and IRB review and approval are still required. And U-M is committed to exploring process improvements and working toward the goal of reducing the total time to opening a clinical trial in all of these areas.
The ACTA is a straightforward and unambiguous document that clearly sets forth the contractual obligations of both parties, and presents language which — while perhaps not ideal for either party — is acceptable to both. Adoption and use of the ACTA will expedite the contract negotiation process and reduce the time it takes to start up industry-sponsored, multicenter clinical trials.
HOW TO USE THIS AT U-M
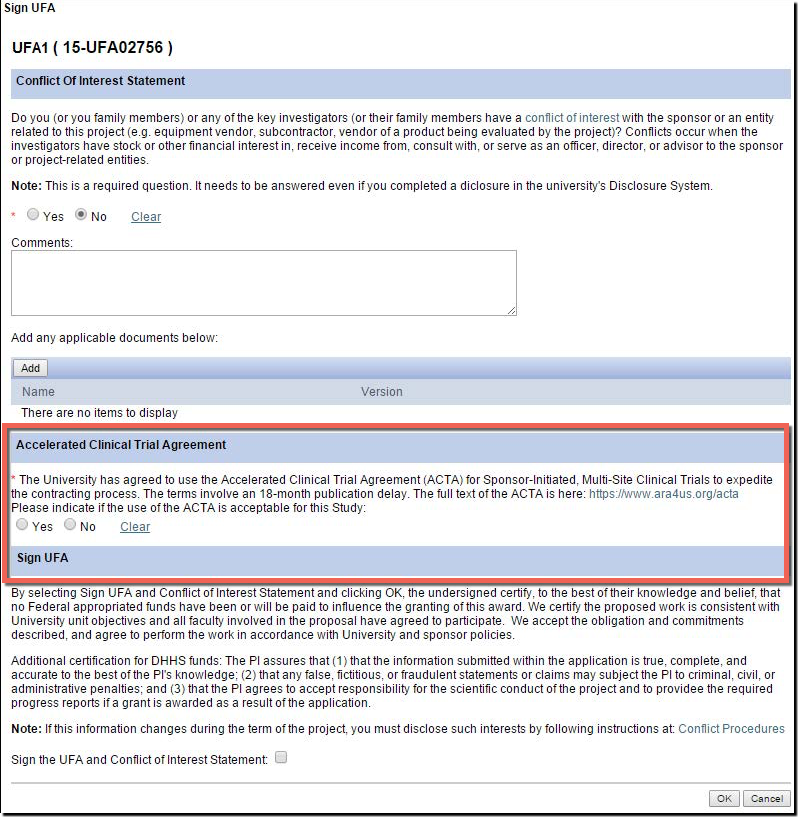
U-M supports and encourage the use of the ACTA for industry-sponsored multi-center clinical trials! Here is what you can do to take advantage:
- There is a new question in the eResearch Proposal Management System (eRPM) for the PI added to the "Sign UFA" activity where you may indicate that you would like to attempt the use of the ACTA during the UFA process.
- Answering “Yes” allows ORSP to approach the sponsor to propose the use of the ACTA.
- Additionally, on the Clinical Trials Routing Form (CTRF), the UFA answer will carry over from the NDA. If not answered, ORSP project representatives can help you later pursue the ACTA.
- Award Notices (AWDs) will also carry language to indicate if the ACTA was accepted.
- Contact one of the following ORSP PRs directly when you have questions, if a Sponsor indicates an interest in using the ACTA, or if a Sponsor would like a template agreement to review.
You can review the language in the agreement from the ACTA Website(link is external). The ACTA is not the promise of a full solution to industry-initiated clinical trial contracting, but it can help reduce one part of the process.
Visit https://umclinicalstudies.org(link is external)/ to learn more about Clinical Trial Studies and participation opportunities.
The CTSUs are trans-departmental business units aligned around common thematics of research. The CTSUs are business units that partner with investigators, their teams, and their departments to ensure the timely and efficient activation and execution of clinical trials at the Medical School. The CTSUs provide the administrative support to allow investigators to focus on the science. More information about the CTSUs is available at the Clinical Trials Support Office website.
The first step is to fill out the CTSU Intake Form. The CTSU will then process your CTA and shepherd your trial through the pre-award processes. The CTSU works closely with the ORSP project representatives (Debra Dill, Lark Haunert, Tricia Haynes, and Mike McAllister) who specialize in clinical trials.