You are here
RPPRs at U-M
I need an eRA Commons ID to submit my RPPR. Where do I start?
Begin the NIH eRA Commons Registration Process
Find Faculty/Staff Registered in NIH eRA Commons
[Kerberos password login may be required]
If the project has Multiple PIs (MPIs), upload the MPI Statement form.
This page provides U-M project teams (research administrators (RAs) and principal investigators (PIs) with the necessary information to complete the required National Institutes of Health (NIH) Research Performance Progress Report (RPPR).
What is an RPPR?
The Research Performance Progress Report (RPPRs) is required at least annually, for NIH-funded studies, to document accomplishments and compliance with terms of the award. All non-competing renewals must submit RPPRs. RPPRs update scientific progress, significant changes, personnel, and plans for the subsequent budget period.
When to Submit an RPPR
- Route RPPRs 2 weeks before the due date to ensure time for the necessary back-and-forth edits, questions, and answers.
- Check ORSP’s instructions to ensure routing goes to the right person in eRPM and Commons.
Who initiates and submits an RPPR?
All RPPRs are submitted to NIH through the eRA Commons online system. Only the PI can initiate the RPPR process in eRA Commons, and then in most cases, an institution’s Signing Official, such as an ORSP Project Representative, must actually submit the completed report to NIH. A more detailed process is outlined below.
The RPPR process requires that progress report submissions are due either 60 or 45 days prior to the new budget period start date.
60 Days
- U or P Grants
45 Days
- R or K Grants
Tips and Reminders for Annual RPPRs

To ensure timely review and submission of the National Institutes of Health (NIH) annual Research Performance Progress Reports (RPPRs) and avoid common mistakes, the Office of Research and Sponsored Projects (ORSP) shares these reminders.
Process to Expect for an NIH Annual RPPR
1. ORSP will provide instructions to the principal investigator (PI) and research administrator (RA) via posted comment on the award record (AWD) 45-60 days before the deadline, indicating who the RPPR should be routed to in eRA Commons and the eResearch Proposal Management System (eRPM).
2. Route RPPRs two weeks before the sponsor due date. This ensures time for the necessary back-and-forth edits, questions, and answers.
3. It's imperative that project teams route to the person specified in ORSP's instructions from the posted comment.
4. In eRA Commons, please route to the person specified in ORSP's instructions from the posted comment.
5. Then, post a comment in the Award record (AWD) to the person specified in ORSP's instructions.
* Please do not route NIH annual RPPRs via an Award Change Request (ACR). Only NIH interim and final RPPRs should be routed via an ACR.
What should a Project Team know about the RPPR process?
- All RPPRs are submitted to NIH through the eRA Commons online system. The Principal Investigator (PI) must initiate the RPPR process in eRA Commons, and an institution’s Signing Official, such as an ORSP Project Representative, must actually submit the completed report to NIH.
- On a monthly basis, ORSP staff members run a list of upcoming RPPRs in eRA Commons. Our staff members alert project teams, via a posted comment on the eRPM AWD record, of any upcoming RPPRs.
- ORSP staff will include our "PHS Key Personnel Annual Reporting: Financial Conflict of Interest Form" requesting information such as a list of current project team staff and other documents that you may need to update as part of the process.
- Complete the RPPR and in eRA Commons, run "Check for Errors" to ensure all issues have been addressed. (If errors exist, ORSP has to return it to the project team, thus delaying the submission.)
- An RPPR may require an update to your Inclusion Enrollment Record. As detailed above, this is now done in NIH ASSIST.
- Once the update is completed in ASSIST, it is necessary to update the status in eRA Commons to "Ready for Submission" (rather than "Work in Progress").
- Post a Comment to the AWD record in eRPM. (If the status in the RPPR is not Ready for Submission, ORSP has to return it to the project team, thus delaying the submission.)
- In eRA Commons, run "Check for Errors" again to ensure all issues have been addressed. (If errors exist, ORSP has to return it to the project team, thus delaying the submission.)
- In eRA Commons, route the RPPR to the requester (i.e., the ORSP staff member who sent it to you) for review.
- Return the completed form(s) via an attachment to the "Post a Comment" activity in the AWD Record in eRPM.
- Use “Request RPPR Review” in the subject line for your Post a Comment
- Begin your reply with “REQUEST RPPR REVIEW” in the text box, in all caps.
- Once these steps are completed, ORSP will review and submit the RPPR.
Troubleshooting
-
Project Team has submitted the Key Personnel Form. In this case, you should search in eRA Commons to see if the RPPR was routed to the wrong person for review. eRA Commons – Route to ORSP Staff Reviewer
-
In eRA Commons, click RPPR and then search for applications with a Status of “Reviewer Work in Progress.”
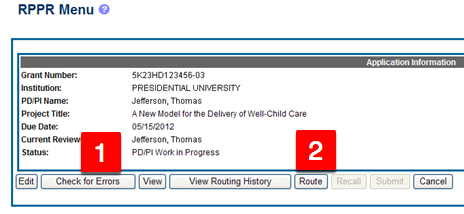
- Click the Grant Number to view the Application Information. eRA Commons – Application Information
- Click Route and select the appropriate ORSP Staff person’s name to assign them as the reviewer, or click Recall to assign the application to yourself. Note: RPPRs that have been assigned to you display in your RPPR Inbox.
- Click Check for Errors. If any errors display, continue with step 5. If no errors display, skip to Phase III.
ORSP gets the email alert from eRA Commons and then ORSP goes into the eResearch Proposal Management System (eRPM) to see if the project team has returned its Key Personnel Form (short version) and contacts the team if the form is not returned or the form is incomplete.
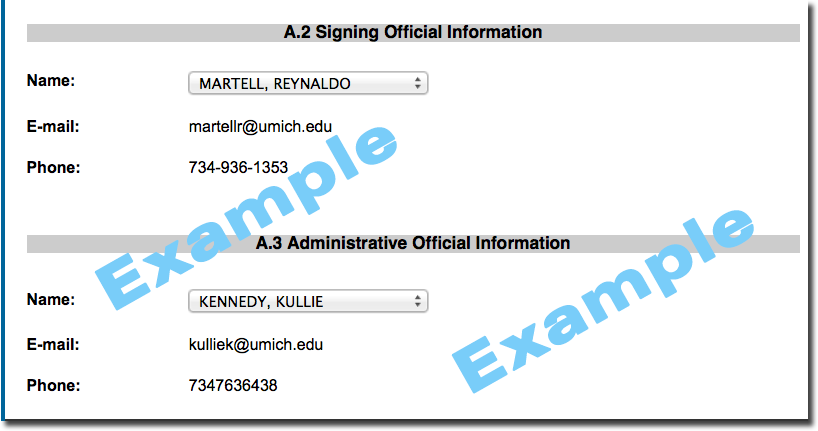
On the cover page, whom should I include as Officials?
- Signing Official (choose your ORSP Project Representative)
- Administrative Official (choose the ORSP Project Representative from your Award record (AWD)
Special Reporting - Performance Sites
- Note the Congressional District and our address.
- Each performance site should only be listed one time.
FAQs
The timing is based on your award date and type. For your specific award-type (SNAP, Non-SNAP, Fellowship or MYF), the due date is reflected in the RPPR Instruction guide, near the beginning, under the RPPR Due Dates section. Start in eRA Commons to begin your report
Yes. NIH offers a comprehensive RPPR Instruction guide.

- Signing Official (choose the ORSP Project Representative from your PAF)
- Administrative Official (choose the ORSP Project Representative from your PAF)
Please remember to include the correct effort. Only place "0" (zero) if applicable to award type (i.e. T32) and according to the NOA from the sponsor.
NIH's xTrain is a part of the eRA Commons module required for all training grant appointments. This is where PIs, university administrators, and trainees:
-
electronically process and submit appointment forms (the PHS 2271 Statement of Appointment form) and termination notices (the PHS 416-7 Termination Notice) associated with institutional research training grants.
- electronically prepare and submit T32 and T35 Statements of Appointments, and T32, T35, F31, and F32 Termination Notices.
Reports handled in eRA
- Internet Assisted Review (IAR): The ability for a PI as a reviewer to submit critiques and preliminary scores for applications being reviewed.
- Financial Status Reports (FSR): The ability for financial report filing via the Commons by the University's Sponsored Programs Office. Financial reports are submitted automatically by Financial Operations.
- Closeout reports: The ability to submit final closeout reports electronically for grants that have ended. NIH closeout reports and questions should be addressed to [email protected].
The eRA Commons (electronic research administration commons) website, by the National Institutes of Health (NIH), that provides principal investigators and their research staff with online features and tools including the abilities to:
- View a full application
- Review current status of an application
- Check for errors
- View application assignment information
- Obtain PDF copies of applications
- Access copies of previous Notices of Grant Award
Get current information on the program official and the grants management specialist
More information from ORSP about using Grants.gov through the U-M eResearch Proposal System
If you have already consulted the helpful NIH Public Access site, and are still unable to come into compliance, the U-M Library staff may be able to assist. Send an email request for assistance to: [email protected].
Yes, but only if any of the investigator's active support has changed. In that case, submit complete Other Support, including both active and pending sources of support and any supporting documents.
Investigators do not need to submit Other Support at RPPR if the only thing that has changed is their pending support.
Yes. Submit complete Other Support including active and pending sources of support and any required supporting documentation.
References and Resources
- NIH's Research Performance Progress Report (RPPR) Guide A link to the current NIH and Other PHS Agency Research Performance Progress Report (RPPR) Instruction Guide, and other instructional resources.
- NIH electronic Research Administration (eRA) Guides and Documentation Excellent support for eRA Commons resources including: Just-in-Time, ASSIST, IAR, RPPR, FCOI, FFR, iEdison (Invention Reporting).
- U-M NIH Access Policy for Publications (ORSP Link) Information on the NIH Public Access Policy and the use of the My NCBI's My Bibliography for managing eRA Commons and for complying with the Policy.
- NIH Extramural Nexus The NIH Extramural Nexus provides regular updates on NIH grants policies and activities that affect the entire grants community. Articles by month.
- NIH xTrain (eRA Commons)
- Key Personnel Form
- nih-progress-report-notice
Questions?
Questions about RPPRs: Contact your ORSP Government Team Project Representative.
eRA Commons registration questions: [email protected]
Questions about xTrain: Address to [email protected] (a Finance-Sponsored Programs address for their who work specifically on the NIH NRSA training grants and fellowships.)